Simple Change, Big Impact: USU Chemists Boost Green Battery Capability
By Mary-Ann Muffoletto |
Members of USU Chemistry and Biochemistry's Liu Lab report advances in sustainable battery design in the journal 'Joule.’ Their research is supported by a USTAR University Technology Acceleration Grant.
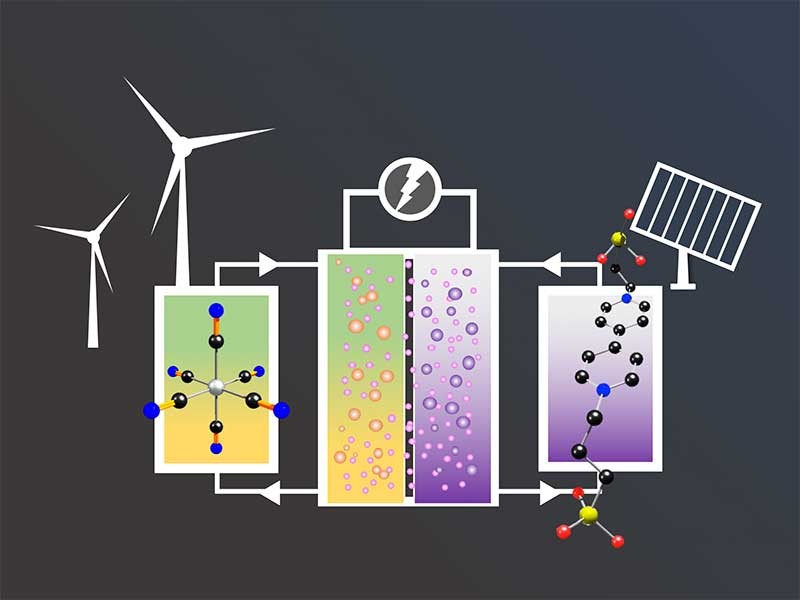
Solar and wind energy are widely regarded as sustainable, environmentally friendly alternatives to fossil fuels, but each is only intermittently available. Both solutions need affordable, high performance energy storage technologies to be considered for widespread, reliable use.
Aqueous organic redox flow batteries, known as “AORFBs,” offer a promising large-scale energy storage solution, but still have limitations. In a molecular engineering study published online October 25, 2018, in Joule, Utah State University chemists report advances to address these limitations.
USU postdoctoral researcher Jian Lu and doctoral student Bo Hu, lead authors of the paper, with graduate students Camden DeBruler, Yujing Bi, Yu Zhao, Bing Yuan, Maowei Hu and Wenda Wu and faculty advisor Tianbiao (Leo) Liu, corresponding author, and with colleagues from the Ocean University of China and Qingdao University of Science and Technology, report a strategy that boosts AORFB storage capacity, safety and performance with a simple design tweak.
The team’s research is supported by USU and a Utah Science Technology and Research (USTAR) Initiative University Technology Acceleration Grant (UTAG).
“We previously found that K4[Fe(CN)6] is chemically stable in pH neutral solution, but not in alkaline solutions,” says Liu, assistant professor in USU’s Department of Chemistry and Biochemistry. “However, the relative low solubility of K4[Fe(CN)6] (0.76 M) is a challenge for flow battery applications.”
In this paper, he says, the team reports a simple formula substitution that significantly improves the solubility of the potassium ferrocyanide, K4[Fe(CN)6], by replacing the potassium cations (K+) with more hydrophilic ammonium ions (NH4+).
“The newly designed (NH4)4[Fe(CN)6] as a cathode electrolyte can achieve a high solubility of 1.6 M in water, twice as that of K4[Fe(CN)6].” Lius says. “In addition, (NH4)4[Fe(CN)6], with its high solubility, also displays much higher conductivity, which increases energy efficiency and power performance for flow batteries.”
Moreover, he says, the team found the charge transfer, using ammonium, is faster than potassium, which further enhances the batteries’ energy efficiency and power performance. When paired with a viologen anode electrolyte called (SPr)2V, a process on which the team recently published, a 24.1 Wh/L (NH4)4[Fe(CN)6]/(SPr)2V flow battery delivered unprecedented cycling stability for 1000 cycles, representing the most stable flow battery known to date.
“This battery also delivered a high power density of 72.5 mW/cm2.” Liu says. “With its low cost materials, this high-performance flow battery is highly attractive for practical energy storage applications.”
USU's Liu Lab reports a strategy that boosts aqueous organic redox flow battery capacity, safety and performance with a simple design tweak. The design breakthrough advances energy storage capabilities for wind and solar power. Credit: Tianbiao Liu.
WRITER
Mary-Ann Muffoletto
Public Relations Specialist
College of Science
435-797-3517
maryann.muffoletto@usu.edu
CONTACT
Tianbiao (Leo) Liu
Assistant Professor
Department of Chemistry and Biochemistry
435-797-2267
leo.liu@usu.edu
ADDITIONAL RESOURCES
TOPICS
Research 878stories Sustainability 145stories Chemistry 110stories Energy 102stories USTAR 64stories Solutions 63storiesComments and questions regarding this article may be directed to the contact person listed on this page.